
- SERIAL DILUTION VS PARALLEL DILUTION SERIAL NUMBER
- SERIAL DILUTION VS PARALLEL DILUTION CODE
- SERIAL DILUTION VS PARALLEL DILUTION PLUS
SERIAL DILUTION VS PARALLEL DILUTION PLUS
Plus the ONLY production camera ever made in classic Zeiss Contax Rangefinder. Some spill over from the Kievs, the Soviet copy of the Contax II/III can also be expected. Zeiss Contax Forum for the classic Zeiss Contax I, II, III, IIa, IIIa, G series, and if you want to push it, the nice Contax point and shoots.
SERIAL DILUTION VS PARALLEL DILUTION SERIAL NUMBER
A sample of this sort of serial number is “Q 94031 *” This article breaks out some.

In the years immediately after World War II, many Zeiss Ikon folding cameras had an additional character attached to the serial number impressed onto the leather covering of the camera.
SERIAL DILUTION VS PARALLEL DILUTION CODE
Keep in mind the 'one letter five numbers' code was used for all Zeiss-Ikon cameras (not only for Contax) so these codes do not indicate total Contax production.
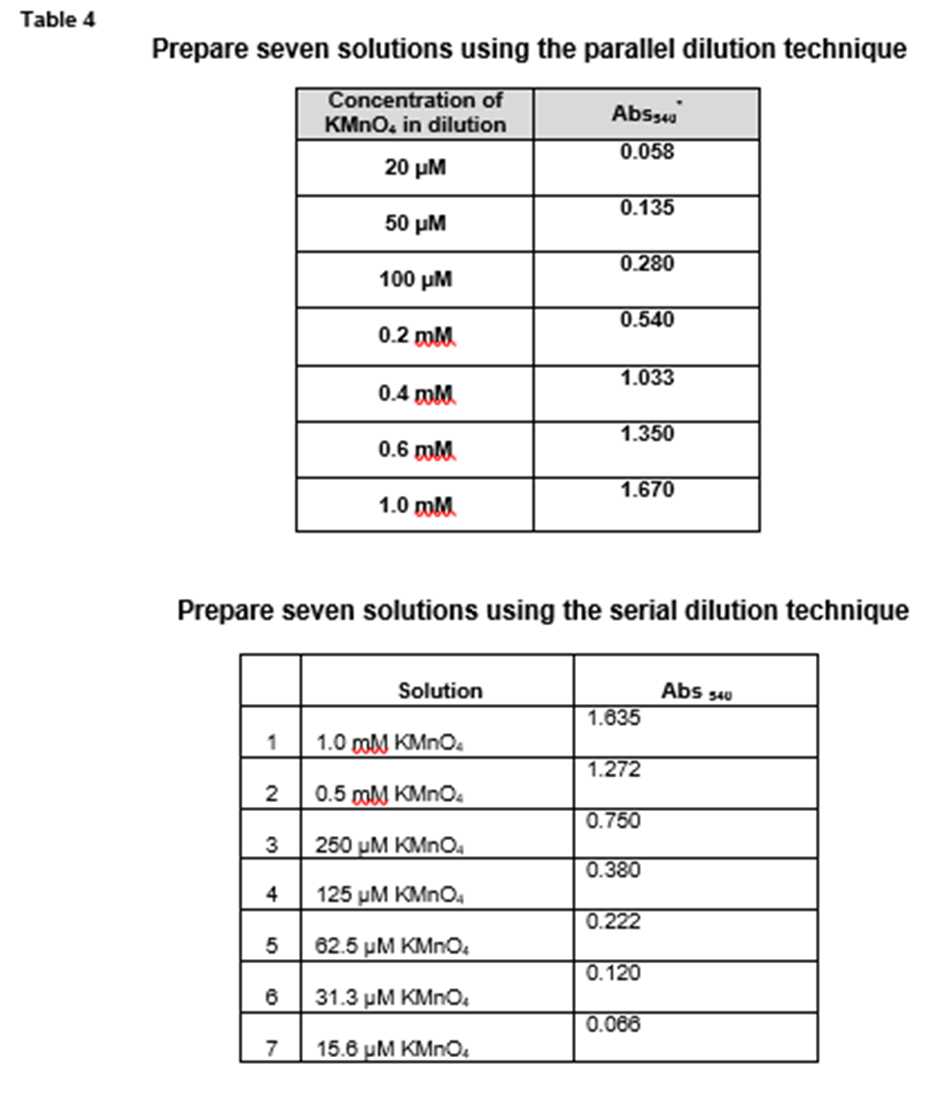
With the Contax II and III, Zeiss not only had a legitimate competitor to Leica, in many ways it bettered Leica and was often considered THE. Therefore, this series has a constant dilution factor of 10. And for the fourth dilution 2 mM divided by 0.2 mM equals 10. For the third dilution, 20 mM divided by 2 mM equals 10. For the second dilution, 0.2 M divided by 20 mM equals 10. For example, for the first dilution 2 M divided by 0.2 M equals 10. To calculate the dilution factor for each dilution, divide the concentration of the starting solution by the concentration of the diluted solution. The factor by which each solution is diluted compared to the previous one is called the dilution factor. Notice that the concentration of each solution is 1/10 th the concentration of the previous solution in the series. There are two situations where serial dilutions should be used rather than parallel dilutions: FIRST: Use a serial dilution when you need several solutions of the same solute and there is a constant dilution factor.įor example, suppose you have a 2 M stock solution of KMnO 4 and you want to make 15 mL of each of the following concentrations of KMnO 4: 0.2 M, 20 mM, 2 mM, and 0.2 mM. This procedure is called the serial dilution technique. Repeated 'serial' dilutions will be necessary to.īIOL 1406 PreLab 2.5 When do I use the serial dilution technique instead of the parallel dilution technique? Another way to make dilutions is to use some of your existing stock solution to make a dilute solution, then use some of the dilute solution to make an even more dilute solution, then use some of that solution to make an even more dilute solution, and so on.

Students are given an unknown dye solution and asked to determine its concentration by comparing it with standards they create. SUMMARY: Building on what was learned in SIMPLE DILUTION, students will determine the best dilution strategy to solve a dilution problem. 13 min - Uploaded by Jennifer HerrellUse this narration in conjunction with the Lab 2 Part 2 Lecture slides and the Lab 2 Prelab.


 0 kommentar(er)
0 kommentar(er)
